Steel is an alloy of iron and other elements, principally carbon. When carbon is the primary alloying element, its content in the steel is between 0.002% and 2.1% by weight. The following elements are always present in steel: carbon, manganese, phosphorus, sulfur, silicon, and traces of oxygen, nitrogen and aluminum. Alloying elements intentionally added to modify the characteristics of steel are: manganese, nickel, chromium, molybdenum, boron, titanium, vanadium and niobium.[1]
Carbon and other elements act as a hardening agent, preventing dislocations in the iron atom crystal lattice from sliding past one another. Varying the amount of alloying elements and the form of their presence in the steel (solute elements, precipitated phase) controls qualities such as the hardness, ductility, and tensile strength of the resulting steel. Steel with increased carbon content can be made harder and stronger than iron, but such steel is also less ductile than iron.
Alloys with a higher than 2.1% carbon content are known as cast iron because of their lower melting point and good castability.[1] Steel is also distinguishable from wrought iron, which can contain a small amount of carbon, but it is included in the form of slag inclusions.[citation needed]
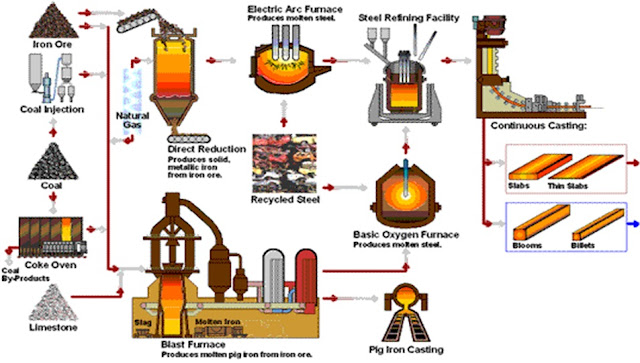
Though steel had been produced by various inefficient methods long before the Renaissance, its use became more common after more efficient production methods were devised in the 17th century. With the invention of the Bessemer process in the mid-19th century, steel became an inexpensive mass-produced material. Further refinements in the process, such as basic oxygen steelmaking (BOS), lowered the cost of production while increasing the quality of the metal. Today, steel is one of the most common materials in the world, with more than 1.3 billion tons produced annually. It is a major component in buildings, infrastructure, tools, ships, automobiles, machines, appliances, and weapons. Modern steel is generally identified by various grades defined by assorted standards organizations. - (Source :Wikipedia)

No comments:
Post a Comment
Note: only a member of this blog may post a comment.